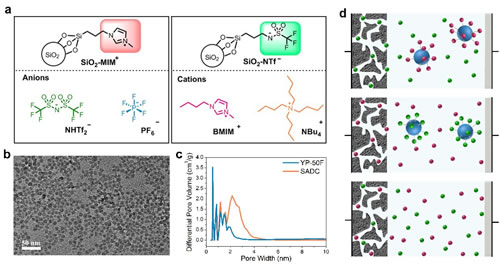
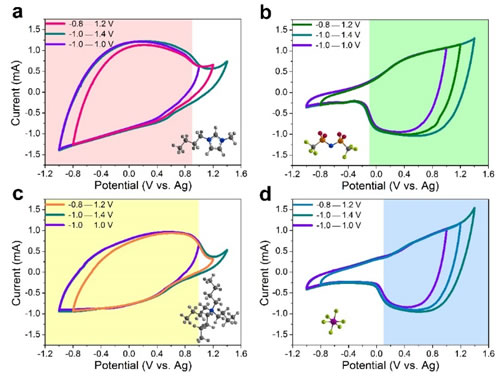
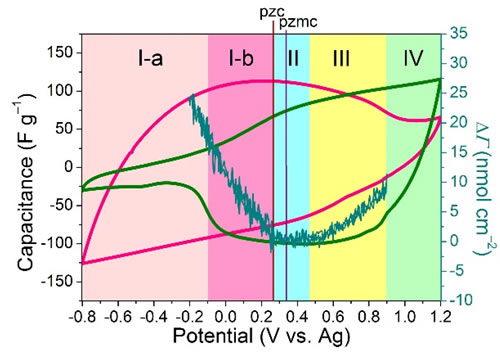
The electric double layer capacitor (EDLC) based on porous activated carbon material and ionic liquid electrolyte has the advantages of rapid charge and discharge, good cycle stability and wide working voltage window, and is a very promising electrochemical energy storage device. Study the energy storage mechanism of EDLC in ionic liquids, especially the mechanism of the influence of the intrinsic structure of ionic liquids on the capacitive properties of porous activated carbon, and reveal the mechanism of energy storage from the microscopic level, properly select ionic liquids, and then construct reasonably. High-performance EDLC has important guiding significance. Recently, the team of the Clean Energy Chemistry and Materials Laboratory of the Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, has made significant progress in the study of ionic liquid energy storage mechanism of EDLC. The researchers prepared four kinds of nano-silica-grafted ionic liquids, and used the characteristic of allowing only one ion of ionic liquid to enter and leave the active carbon channel during the charge and discharge process, and achieved the purpose of analyzing the anions and cations, respectively. The results can provide new strategies for studying the energy storage behavior of ionic liquids in EDLC. The structural characteristic of silica-grafted ionic liquids is that an ion (cation BMIM+, NBu4+, or anion NTf2–, PF6–) is free; and the counter-charged ion that acts as an equilibrium charge: trifluoromethanesulfonimide Anions (NTf–) and methylimidazole cations (MIM+) are covalently bonded to silica nanoparticles with a size of 7 nm. The pore size of most of the pores of activated carbon materials selected in this study was less than 4nm, so that the ions connected to silica were blocked outside the active carbon channels, while the free ions to be measured (cations BMIM+, NBu4+, or anions NTf2–, PF6–) could pass Hole. On this basis, a simple electrochemical test can realize the quantitative analysis of ions entering the free channel, that is, using the current of the cyclic voltammetry curve to directly reflect the capacity contributed by the ion. Based on the above method, the research team found that with commercial activated carbon YP-50F as the electrode, the capacity of the cations BMIM+, NBu4+ and anions NTf2–, PF6–, and the specific voltage window for each ion contribution capacity can be characterized. Using quartz crystal microbalance (EQCM), the researchers further characterized the energy storage mechanism of activated carbon YP-50F in ionic liquid (BMIM-NTf2), combined with the respective electrochemical properties of BMIM+ and NTf2 – to deepen the mechanism of energy storage. Hierarchical explanation. The relevant research results were published in Nature-Communications. The study was funded by the National Natural Science Foundation of China and Lanzhou Institute of Chemical Industry for "1-3" key projects. Figure 1. Strategy diagram. a: Silica graft ion and free ion structure diagram; b: Silica transmission electron micrograph; c: Activated carbon pore size distribution diagram; d: Energy storage diagram of activated carbon in three different electrolytes, from top to bottom It is anion free, cation fixed, cation free, anion fixed, cation and anion are all free Figure 2. Cyclic voltammograms of activated carbon YP-50F electrode in four different electrolytes. ad is SiO2-IL-BMIM, SiO2-IL-NTf2, SiO2-IL-NBu4, SiO2-IL-PF6 Figure 3. Detailed study of the properties of the combined anion and cations and the energy storage mechanism of quartz crystal microbalance experiments. Small Folding Knife,Folding Coin Knife,Folding Mini Knife,Bullet Shape Pocket Knife Easy-Go Outdoor Co.,Ltd. , https://www.yjeasy-go.com