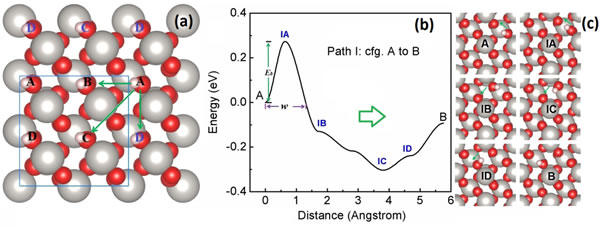
Recently, Yang Yong, a researcher at the Institute of Solid State Physics, Chinese Academy of Sciences, and Professor Kawazoe of Tohoku University, Japan, based on first-principles calculations for the adsorption and diffusion of hydrogen atoms on the beta-PtO2 (001) surface and nuclear quantum effects Systematic research. Related papers were edited and published online in the form of ASAP (As Soon As Publishable) Article in The Journal of Physical Chemistry C (J. Phys. Chem. C, DOI: 10.1021 / acs.jpcc.9b04107). The adsorption and diffusion of hydrogen on the surface of solid materials plays an important role in some basic physical and chemical processes, such as the electrode reaction of oxyhydrogen fuel cells, the synthesis and decomposition of hydrocarbons, the hydrogen storage process, and metals and alloys Hydrogen embrittlement process. In the past few decades, a lot of research has been done on the adsorption and diffusion of hydrogen on metals, especially transition metal surfaces. In contrast, little is known about the process of hydrogen adsorption and diffusion on transition metal oxide surfaces, although the latter has important applications in the fields of catalysis and energy conversion materials. Taking a proton-exchange membrane fuel cell as an example, after hydrogen ions reach the cathode, they first recombine with electrons arriving via wires to form hydrogen atoms, and then react with O2 adsorbed on the cathode surface to produce water. It was experimentally found that after being used for a period of time, the platinum electrode surface of the battery will be covered with a layer of oxide whose main component is beta-PtO2. A natural question is, how does the formation of this layer of oxide affect the hydrogen transport process on the electrode surface? On the other hand, compared to other elements such as oxygen, the mass of hydrogen is very small; therefore, the quantum motion of the atomic nucleus, the nuclear quantum effect (Nuclear Quantum Effects), will be more obvious. So, what role will nuclear quantum effects play in the diffusion process? To this end, based on first-principles calculations, the researchers systematically studied the adsorption and diffusion of hydrogen atoms on the beta-PtO2 (001) surface and nuclear quantum effects. Calculations show that when hydrogen atoms diffuse along certain specific paths (Figure 1), the potential barrier to be overcome is comparable to that on the surface of the Pt electrode. Therefore, it can be predicted that even after the formation of oxides, the transport of hydrogen on this surface is almost unaffected. Next, based on the quantum mechanics Wentzel–Kramers–Brillouin (WKB) approximation and the latest achievements of statistical mechanics on particle kinetic energy distribution, Yang Yong gave the classical and quantum probabilistic analytical expressions of single particles crossing the barrier at a specific temperature . Compared with the path integral molecular dynamics, a theoretical tool commonly used to study nuclear quantum effects, the advantage of this new method is that it can use a relatively small amount of calculation under the premise of ensuring reasonable accuracy Research on nuclear quantum effects in large-scale atomic and molecular systems such as surfaces and body states. Applying this method to the diffusion process of hydrogen atoms in the beta-PtO2 (001) plane, the classical probability (PC) and quantum probability (PQ) of hydrogen atoms crossing a given barrier can be calculated. As shown in Figure 2, at room temperature (~ 300 K), the quantum probability and the classical probability are comparable in magnitude and cannot be ignored; while at low temperatures, the quantum probability is much larger than the classical probability, occupying an absolutely dominant position. This research was supported by the National Natural Science Foundation of China. Figure 1. (a) Schematic diagram of hydrogen atom diffusion in the β-PtO2 (001) plane; (b) optimal energy path for diffusion from configuration A to configuration B; (c) correspondence from configuration A to configuration B Some intermediate adsorption state. Figure 2. During the diffusion of hydrogen atoms from configuration A to configuration B along the optimal energy path, the classical crossing probability (PC) and quantum crossing probability (PQ) change with temperature. Regular LED Grow Light 150W,Square 150W LED Grow Light,LED Grow Light Panel,150W Grow LED Lights Yichang Green Eco-agriculture Co., Ltd. , https://www.plantgrowlamp.com